POC Test
Seinda’s series of point-of-care testing solutions:
– Ocular Inflammation (MMP9-POC Test)
– Dry eye (LTA-POC Test)
– Allergy (IgE-POC Test)
– IL6-POC Test and IL8-POC Test (Launching in 2026)

Seinda’s series of point-of-care testing solutions:
– Ocular Inflammation (MMP9-POC Test)
– Dry eye (LTA-POC Test)
– Allergy (IgE-POC Test)
– IL6-POC Test and IL8-POC Test (Launching in 2026)

Fast Turnaround: Quantitative results within 10–15 minutes at the point of care.
High Sensitivity: Accurately detects tear IgE levels, even at low concentrations, allowing for early identification of allergic responses.
Small Sample Volume: Requires only 1.1 or 2.2 µL of tear fluid, ensuring patient comfort and efficient workflow.
Barcode-Linked Protocol: Each cassette includes a unique barcode, allowing the analyzer to automatically select the correct test settings—no manual calibration or software updates required.
Integrated Safety and Quality Control: Built-in validation and system checks ensure test reliability and reproducibility across every clinical setting.
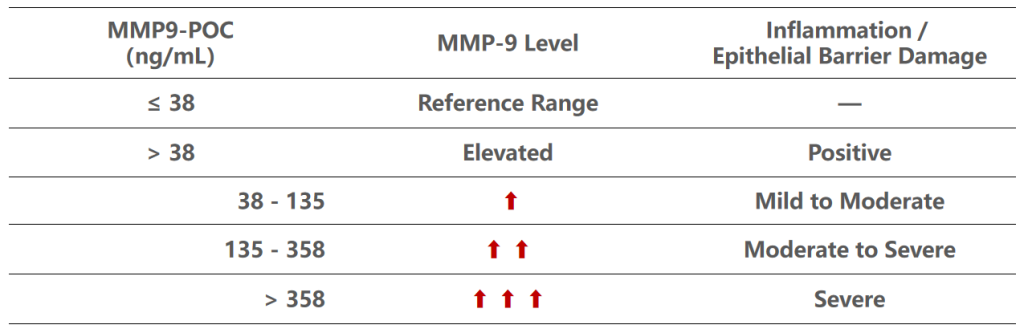
Matrix Metalloproteinase-9 (MMP-9) is an enzyme linked to the breakdown of corneal epithelial barrier function and inflammation in DED. MMP-9 is a critical biomarker associated with ocular surface inflammation in Dry Eye Disease (DED). Elevated levels are associated with more severe disease and poorer ocular surface health.
Used with the i-ImmunDx™ Analyzer, the MMP-9-POC test is a quantitative point-of-care assay designed to detect Matrix Metalloproteinase-9 (MMP-9) in tear fluid. The test provides clinicians with a rapid and objective tool to assess inflammatory status, improving diagnostic precision and treatment guidance.
MMP9-POC Clinical Applications:
– Discriminate Inflammatory DED vs Non-inflammatory DED;
– Treatment Selection: prescribe anti-inflammatory treatment or not and for how long;
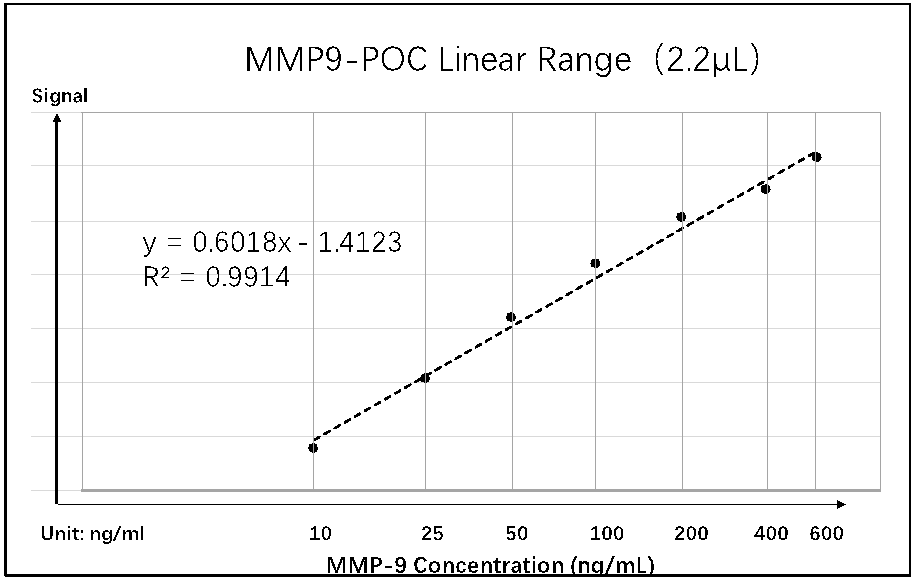
MMP9-POC Analytical Properties:
Measurement of Ocular Surface Inflammation/Damage
For more information, please contact us at sales@seindabio.com.
MMP9-POC Brochure
Publications
Lymphotoxin-α (LTA) plays a pivotal role in maintaining ocular immune tolerance by activating regulatory T cells (Tregs) through the TNFR2 pathway. Reduced LTA levels in tears have been associated with disrupted immune homeostasis, a key factor in the pathogenesis of DED. By quantifying LTA, the LTA-POC test aids in identifying patients with immune-mediated DED, supporting personalized treatment strategies and monitoring therapeutic efficacy.
Clinical Significance:
LTA-POC: Quantifying Immune Balance in Dry Eye Disease
Used with the i-ImmunDx™ Analyzer, the LTA -POC test is a quantitative point-of-care assay designed to quantify LTA in tear fluid. The test provides clinicians with a rapid and objective tool to assess inflammatory status, improving diagnostic precision and treatment guidance.
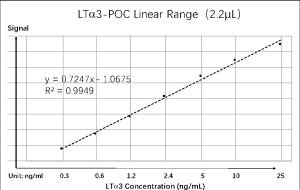
LTA-POC Analytical Properties :
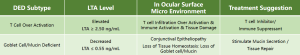
Why Choose LTA-POC:
Traditional diagnostic methods for DED often lack specificity and fail to correlate with disease severity. The LTA-POC test addresses this gap by providing objective, molecular-level data on immune status, enabling clinicians to differentiate between DED subtypes and tailor interventions accordingly.
For more information, please contact us at sales@seindabio.com.
LTA-POC Brochure
Publications
Immunoglobulin E (IgE) is a specific mediator of type I hypersensitivity reaction and plays a key role in the pathogenesis of allergic conjunctivitis. It has important guiding significance for clinical diagnosis and treatment. An increase in Tear IgE is helpful for the diagnosis of allergic conjunctivitis.
The IgE-POC is a point-of-care diagnostic test developed to measure total Immunoglobulin E (IgE) levels in tear fluid—offering a rapid, objective solution for the detection and assessment of allergic conjunctivitis.Designed for use with the i-ImmunDx™ Analyzer, this test enables clinicians to confirm allergy-related inflammation in minutes, improving diagnostic accuracy and supporting more targeted treatment decisions.
Clinical Significance:
Allergic conjunctivitis can be difficult to distinguish from other forms of ocular surface inflammation based on symptoms alone. Used with the i-ImmunDx™ Analyzer, the IgE-POC test is a quantitative point-of-care assay designed to quantify IgE in tear fluid. The test provides molecular-level confirmation, helping clinicians differentiate allergic from non-allergic causes of eye irritation and enabling more precise treatment planning—including appropriate use of antihistamines or anti-inflammatory therapies.
Diagnosis of AC, discriminate from infection or Dry Eye
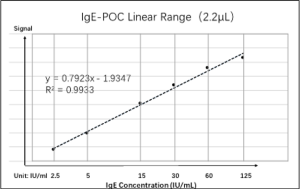
IgE-POC Analytical Properties:
Why Choose IgE-POC:
Guidelines: Tear IgE measurement for the diagnosis of allergic conjunctivitis
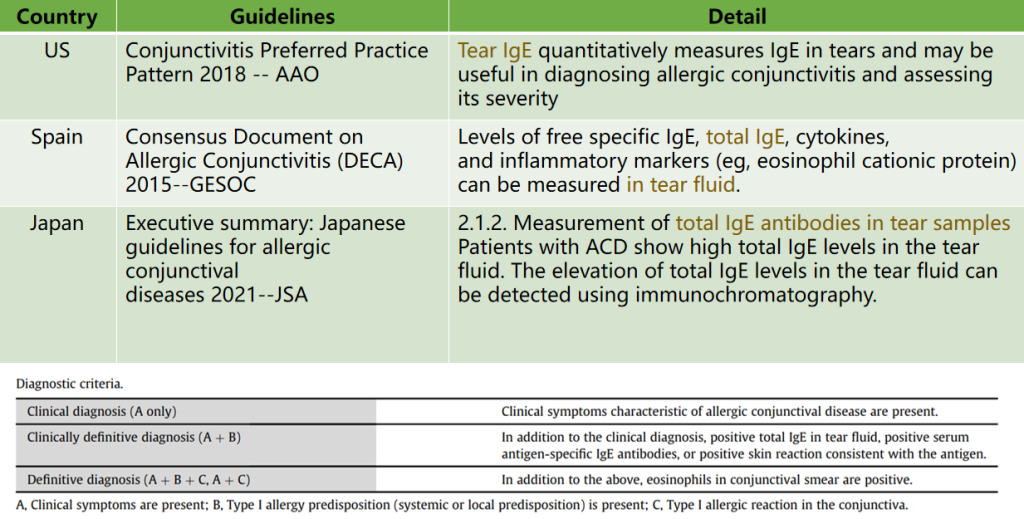
For more information, please contact us at sales@seindabio.com
IgE-POC Brochure
Launching in 2026
Launching in 2026